|
|
|
|
Contact:
Carbon Monoxide Hazards with Backpacking Stoves

Carbon monoxide (CO) poisoning in tents is a real danger for those that use lanterns or stoves in tents and snow caves. CO is an odorless, tasteless, colorless, nonirritating gas formed by the incomplete combustion of stove fuels and has a way of sneaking up on unsuspecting campers. This poisonous gas can cause minor symptoms such as headache, nausea and fatigue, but can also result in long-term cognitive impairment or death. Between 1990 and 1994, there was an annual reported average of 30 fatal CO poisonings in tents or campers in the USA. And considering the frequency and amount of deaths caused by using stoves in enclosed spaces, it appears that the deadly dangers of using a stove in a tent are not common knowledge.
Disclaimer on the following information -
The following information is based on educated speculation by a non-expert on information from many sources. A good deal of the research supporting the following article is based on experiments done in cardboard boxes and not on actual or realistic outdoors situations. The majority of the supporting documentation on this page seems creditable and authored by respected authorities but these authors may have limited knowledge of stove use, flame theory and little or no outdoors experience. References are provided so that you can personally research this matter further and come to your own conclusions.
If you would like to skip all of the technical mambo jumbo and get straight to the skinny - the Recommendations for Avoidance are straight and to the point.
Your body uses blood to transport and remove various nutrients and waste products to and from the tissues of your body. One of the most important aspects of blood is it's ability to continuously deliver oxygen. This is done with red blood cells that are packed full of special proteins called hemoglobin. The hemoglobin are designed to "grab" on to oxygen molecules as they diffuses across the pulmonary capillary membrane in the lungs. It then holds on to oxygen as the blood flows through the body and is able to unload it where needed in your body.
CO can rapidly diffuse across the pulmonary capillary membrane in the lungs and like oxygen binds to hemoglobin. CO in fact is able to bind with hemoglobin about 200-250 times better than oxygen. When hemoglobin binds with CO it forms a complex called carboxyhemoglobin (COHb). And the amount of carboxyhemoglobin in your blood determines your level of carboxyhemoglobinemia (also referred to as COHb), the level of total CO bound to red blood cells. Non-smokers can have as much as a 3% COHb under normal environmental conditions, while smokers may have as much as 10-15%.
When CO binds to an oxygen binding site on hemoglobin it changes the shape of the other three oxygen binding sites on that protein, which makes it very difficult for oxygen to attach to it and to off-load oxygen to peripheral tissues. This drastically impairs the amount of oxygen that is delivered to your tissues and brain. The higher your COHb, the lower the amount of oxygen that can be delivered to your brain, heart and other tissues.
To make matters worse, about 10-15% of the CO in your body will bind to other molecules in your body such as myoglobin, cytochromes, and NADPH reductase. CO will stay bound to these molecules much longer than it does to hemoglobin and particularly affects the function of your body's cells by impairing oxidative phosphorylation at the mitochondrial level. Because of this, high oxygen demand organs (i.e. heart, brain and lungs) with bound CO will be unable to perform at optimal levels even with clearing of CO from the blood and with good oxygen flow.
CO poisoning also may cause several other problems such as reperfusion injury from partially reduced oxygen species produced during hyperperfusion. Xanthine dehydrogenase is converted by CO to xanthine oxidase, which produces damaging oxygen radicals that basically raise hell in cells. Guanylyl cyclase is activated by CO to produce cyclic GMP, and nitric oxide synthase, which makes NO that in turn appears to affect regulation of diameter in resistance vessels and therefore causes perfusion problems.
A very small portion of CO (less than a percent) will remain in gaseous from in the blood and is believed to contributes to the neurocognitive manifestations of CO poisoning with possible direct damage to brain tissues. CO acts as a gaseous neurotransmitter that affects respiration rate, heart rate, vasodilation, learning, memory and long-lasting adaptation to sensory stimuli (esp. odors). Exposure to CO can also cause reversible demyelinization of central nervous system lipids due to lipid peroxygenation.
The effects of CO toxicity are not short lived. At sea level, it takes 4-5 hours of normal breathing to eliminate half of the CO bound to your hemoglobin and much longer to clear other tissue. At higher altitudes, it takes much longer to breath off the built up CO in your blood. And since the effects of CO accumulation is cumulative, a trip spanning several days can easily lead to severe CO poisoning. There are also problems with long term and delayed toxicity that may last longer than a year.
The effects of CO poisoning varies greatly from person to person and symptoms are largely vague. The lips and skin can appear "cherry red" with high COHb levels, but this is a poor assessment tool to use.
The follow table shows some of the symptoms associated with certain serum levels of COHb, and real life situations will vary greatly from person to person and situation to situation.
General Effects of Various COHb levels at sea level
|
|
0-10% |
Generally does not cause symptoms for healthy folks. |
|
|
10-20% |
Mild frontal headache, malaise, nausea, vomiting, dizziness and loss of manual dexterity |
|
|
20-30% |
Headache with rapid heartbeat, confusion, lethargy, visual disturbances. This level may lead to death as the victim loses both the interest and the ability to leave a danger area (such as fire) |
|
|
30-40% |
Collapse |
|
|
40-50% |
Seizures |
|
|
50-60% |
Coma |
|
|
60-70% |
Death in 2 hours |
|
|
80-90% |
Death in less than 1 hour |
|
|
90-100% |
Death in minutes |
Leigh-Smith S. Carbon monoxide poisoning in tents--a review. Wilderness and Environmental Medicine. 2004 Fall;15(3):157-63. and other sources
The level of CO exposure considered safe varies from guideline to guideline. OSHA in the States now sets the limit at 50 ppm over an 8 hour work day while other countries set limits at 30-35 ppm. The maximum amount of CO allowable in the work area (for short term exposure) is generally between 100-200 ppm, again dependant on regulating agency. The following graph depicts predicted levels of COHb vs. duration of various levels of CO for a sedentary (not breathing hard) male at sea level.
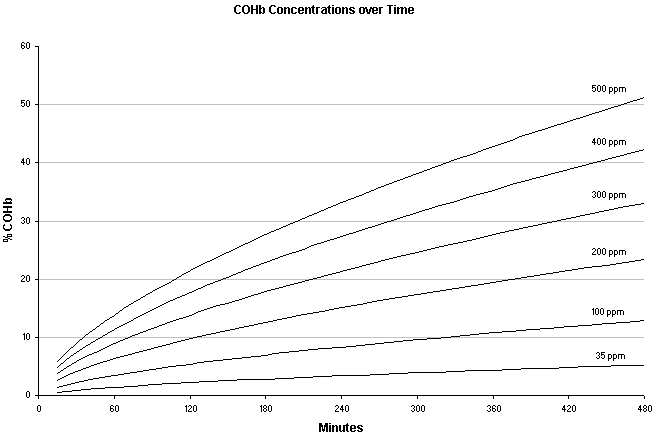
Log(%COHb) = 0.858log[CO] + 0.63log(t) - 2295 (Peterson JE, Stewart RD: Predicted the carboxy-hemoglobin levels result from carbon monoxide exposure. J Appl Physiol 1975;39:633-638.)
This chart is a bit misleading since it appears that the CO concentrations that you are likely to experience in a tent (up to 600 ppm) will only cause nausea and headaches. Note that the amount of COHb at sea level it takes to give you a headache, is the same amount that will cause you to collapse at higher altitudes. It is also important to note that COHb levels are also accumulative and will build up over time with each subsequent exposure to CO.
Delayed Neurologic Sequelae
There is also a risk of delayed neurologic sequelae (DNS) 3-240 days after apparent recovery of 40% of patients with significant CO exposure. DNS includes various degrees of impaired cognition, memory dysfunction, vertigo, ataxia, parkinsonism, muscle rigidity, gait disturbance, disorientation, mutism, urinary incontinence, fecal incontinence, cortical blindness, hearing loss, tinnitus, nystagmus, seizures, coma, electroencephalographic abnormalities, cerebral edema, leukoencephalopathy, diabetes insipidus and globus pallidus necrosis. These deficits may persist for a year or longer.
|
Brain Syndrome associated with Delayed Neurologic Sequelae |
|
|
|
|
|
Psychiatric Symptoms |
% of Patients |
|
Apathy |
100 |
|
Disorientation |
100 |
|
Amnesia |
100 |
|
Hypokinesia |
95 |
|
Mutism |
95 |
|
Irritability, distractibility |
91 |
|
Apraxia |
76 |
|
Bizarre Behaviors - silly smiles or frowning |
70 |
|
Mannersitic Behavior |
41 |
|
Irrational Confabulatory Talking |
30 |
|
Insomnia |
19 |
|
Depressed Mood |
15 |
|
Delusions |
12 |
|
Echolalia |
2 |
|
Elated Mood |
2 |
|
|
|
|
Neurological Symptoms |
|
|
Urinary and/or fecal incontinence |
93 |
|
Gait disturbance |
91 |
|
Glabella sign |
91 |
|
Grasp reflex |
87 |
|
Increased muscle tone |
86 |
|
Retropulsion |
72 |
|
Increased DTR |
22 |
|
Placid paralysis |
19 |
|
Tremor |
14 |
|
Dysarthria |
9 |
Min SK. A brain syndrome associated with delayed neuropsychiatric sequelae following acute carbon monoxide intoxication. Acta Psychiatr Scand. 1986 Jan;73(1):80-6.
Chronic Carbon Monoxide Poisoning
People exposed to low levels of carbon monoxide for long periods can develop chronic CO poisoning, which can be a very serious problem. Fatigue, headache and dizziness are the three most common symptoms, but chronic symptoms also include flu-like illness, headaches, tearfulness, depression, agitation, anxiety, decreased memory, attention and concentration skills, poor reasoning skills, irritability, euphoria, and overall personality changes.
Chronic CO poisoning can be extremely difficult to diagnose and is often confused with chronic fatigue syndrome, multiple chemical sensitivity, fibromyalgia, malingering, anxiety and depression. Since symptoms are vague and COHb levels are often unremarkable, psychometric testing may be required to identify problems. An MRI, CT or SPECT scan may demonstrate abnormalities, but may not demonstrate any problems early on in poisoning.
English Translation of this Section
Carbon monoxide binds to blood cells and cause people to asphyxiate. If you don't die from it right away, carbon monoxide can cause a lot of other long term problems like pooping in your pants.
The higher you go above sea level, the lower the barometric and oxygen pressures become. Since our lungs are designed to utilize the gradient created by the greater oxygen pressures outside of our body than inside, a drop in inhaled oxygen pressures has a detrimental affect on how much oxygen gets in to our blood and to our tissues. A decrease in the amount of oxygen diffusing into the blood, and therefore being transported throughout your body, can have severe effects on high oxygen dependant tissues and organs, such as the brain and heart. To further complicate things, burning a stove in an enclosed space consumes oxygen, which in turn lowers the partial pressure of oxygen and amplifies the decreased oxygen (hypoxia) that may already be present at altitude.
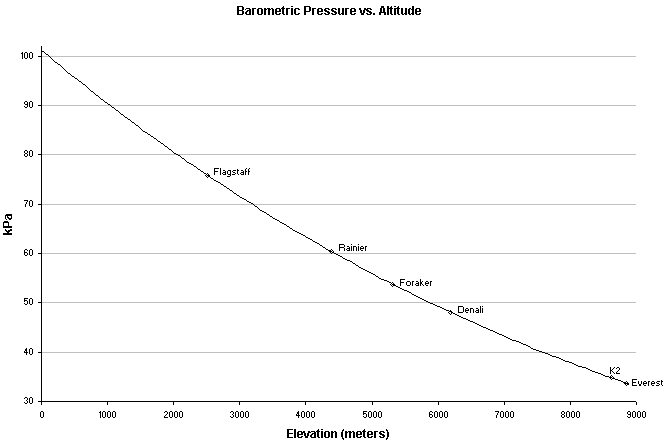
Model Atmosphere equation: PB = exp(6.63268 - 0.1112 h - 0.00149hh), where PB is barometric pressure in Torr and h is altitude in km
It is true that there are people who have summited the highest point of the world without supplemental oxygen, but this is far from the normal abilities of the human body and not without ill and long term consequences - such as the far greater occurrence of death during decent compared to those using oxygen. Even at altitudes of just 10,000 feet (3000 meters), people start to feel the effects of altitude and may suffer from the affects of lack of oxygen (hypoxia). At and above these altitudes, one should be concerned with the many problems directly associated with this drop in atmospheric pressures (i.e. acute mountain sickness, high altitude pulmonary edema and high altitude cerebral edema).
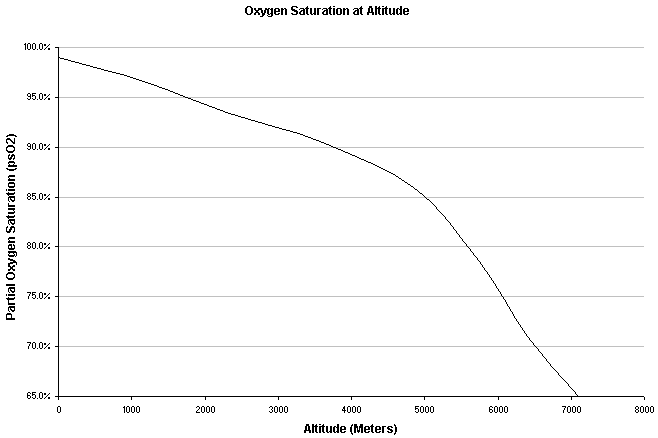
Data Plotted from study of 11male and 2 female acclimated mountain climbers by Tannheimer M, Thomas A, Gerngross H. Oxygen saturation course and altitude symptomatology during an expedition to broad peak (8047 m). Int J Sports Med. 2002 Jul;23(5):329-35.
Since the CO bound to your hemoglobin decreases the amount of available O2 you can pull out of the air, the higher your COHb, the lower your body's blood oxygen level. Even small amounts of CO in your body can have severe affects on your ability to function at higher altitudes. COHb levels that may only cause slightly noticeable problems at sea level can be very deadly at high altitudes. In one experiment (Forbes WH, Sargent F, Houghton FJW. The rate of carbon monoxide uptake by normal men. Am J Physiol. 1945;143: 594-608.), four out of seventeen healthy subjects at a simulated altitude of 4725 meters (15,500') collapsed when their COHb reached between 9-19%.
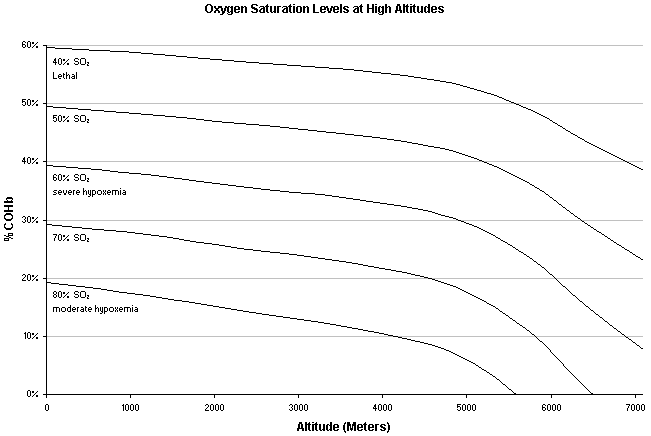
Predicted COHb and O2Hb saturation in regards to altitude calculated from oxygen blood saturation findings of 11male and 2 female acclimated conditioned mountain climbers by Tannheimer M, Thomas A, Gerngross H. Oxygen saturation course and altitude symptomatology during an expedition to broad peak (8047 m). Int J Sports Med. 2002 Jul;23(5):329-35.
Note: graph doesn't take into account several variables such as increased basilar CO production, lack of attitude acclimation, erratic respiratory cycles at high elevations, inability to compensate even with 100% O2 and factors that may only be applicable to high altitudes. In fact, the formula used to make this chart may not reflect real life oxygen saturation levels.
Increases in altitude amplify the dangers of CO exposure several ways. First off, the decreased levels of oxygen pressures at higher altitudes will further decrease the amount of oxygen the blood can hold in a CO taxed system. Decreased oxygen pressures also results in CO needing more time to clear itself from your body. At higher altitudes, you also create more CO and breath faster, which causes you to inhale more CO from a burning lantern or stove.
Those who have been at high altitudes for long periods should develop more red blood cells and hemoglobin and will therefore accumulate more CO than someone not acclimated to higher elevations. This allows for the built up of higher amounts of CO in the blood which increases the half-life of COHb, causing it to linger in the body longer.
Those at high altitudes are also more likely to be subjected to harsh cold (the lack of oxygen adds to this), hazardous precipitation and wind which increased the likelihood that these travelers will zip up their tents or block off ventilation to snow caves. Tents at higher altitudes are also subject to icing over or being covered in snow. All of these factors decrease the ventilation and increase concentrations of CO in shelters. At higher altitudes, climbers may opt to use snow caves in lieu of tents, which provide even worse ventilation than tents. The dehydration and cold experienced in many high altitude settings may also increase the frequency of firing up a stove for a brew or heat. Stoves and lanterns are also oxygen deprived and will burn less efficiently, producing more CO due to incomplete burning of fuel and longer burn times needed to cook or melt snow.
People at high altitudes act weird anyways due to the lack of oxygen and fatigue, and the signs of CO poisoning are often dismissed as just fatigue and/or acute mountain sickness. This is a problem as CO intoxicated individuals may not realize their condition and continue to expose themselves to high levels CO. It's also important to note that it's not only affixation caused by CO poisoning that will kill you. CO poisoning can create various neurological problems and premature fatigue that could cause a climber to fall off of a cliff and plummet to his death, scattering body parts and very expensive mountaineering gear across the white jagged landscape.
One Norwegian study (see Thomassen below) exposed 7 healthy young nonsmoking male subjects to 2 hours of melting snow with an Optimus 111 stove in three different tents at a campsite 200m (650') above sea level . They all ended up with COHb levels of greater than 20%. At that low elevation the subjects were already experiencing signs of CO poisoning from their burning stove and were subject to the possible long term neurologic damage and potentially deadly CO levels. Exposure to similar levels of CO at greater elevations is a sure invitation to a very dreary death.
Since prolonged exposure to low concentrations of CO can result in considerable poisoning, backpacking trips that last just a few days can easily lead to a dangerous rise in blood CO and chronic poisoning. In fact, long term exposure to low concentrations of CO is considered more dangerous that short term exposure to high concentrations of CO.
Sitting in a zipped up tent with a burning stove and knowing the hazards of carbon monoxide poisoning isn't conducive to long life, yet is common practice by many mountaineers. In fact, most of the people who read this information may still opt to use a stove in a tent for whatever reason. So for those who aren't going to subject themselves to cooking outside of their tents in mud puddles or freezing blizzards, there are a few facts they should be aware to help decrease (not eliminate) the risks of CO exposure. Please note that with good common sense practices you can reduce the likelihood of severe problems in the outback, but you may still be subject to long term problems with even small amounts of CO exposure.
Using a stove in a tent is dangerous - do so at your own risk.
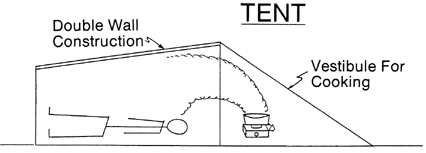
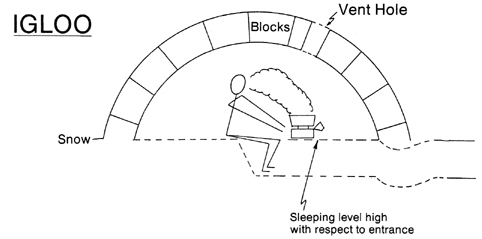
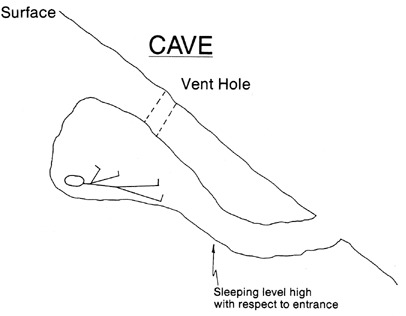
Turner WA, Cohen MA, Moore S, et al. Carbon monoxide exposure in mountaineers on Denali. Alaska Med. 1988; 30:85-90.
One of the easiest ways to decrease CO exposure is through ventilation. This decreases the concentration of CO as well as allow oxygen to enter your tent or shelter. Tents "breath" considerably better than igloos or snow caves, particularly since snow shelters tend to ice over on the inside when using a stove. But tents also lose much of their breathability to if iced over or covered in snow. Obviously, tents made out of vapor barrier material will trap more CO in the tents for longer than other tents. Wind also plays a part in ventilation, particularly with tents. Therefore, CO buildup in tents is much greater in zero-wind conditions.
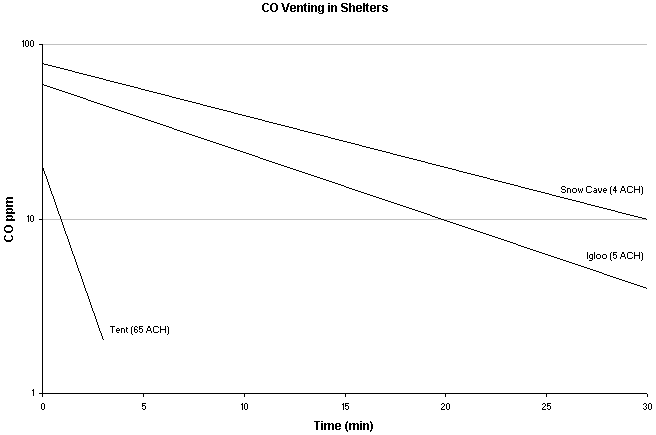
Chart shows CO levels after stove was shut off. ACH- air exchange rate. Tent used was a double walled nylon tent with entrance door half open. Igloo had vent hole ~45cm2. Snow cave had vent hole ~20cm2. Source - Turner WA, Cohen MA, Moore S, et al. Carbon monoxide exposure in mountaineers on Denali. Alaska Med. 1988; 30:85-90.
Research performed on Denali Alaska (Turner WA, Cohen MA, Moore S, et al. Carbon monoxide exposure in mountaineers on Denali. Alaska Med. 1988; 30:85-90.) suggest the beneficial use of a ventilation hole at least the size of a ski pole basket (about 50cm2 or 8in2) located as high as possible and close to the operating stove to bring CO levels to more acceptable levels. It is important to have ventilation high since, contrary to popular belief, CO doesn't pool in shelters but instead rises since it is a hot combustion gas and is less dense than other gasses in the air (molecular weights: CO=28, N=28, O2=32, CO2=44). Other research suggests that it is important to have a oxygen inlet opening low in your tent to prevent decreased oxygen levels which will exponentially magnify CO production by your stove.
Since sitting in your shelter can mask many of the symptoms of CO poisoning, it is recommended that your get up every so often and walk around a bit. This will give you some fresh air, force you to open up the tent and may "reveal" any symptoms you might have. Headaches are a bad sign and if you get up and find you can't walk, you are very close to lethal poisoning.
Another way to decrease your exposure to CO is to not create it in the first place. Let's begin with a brief introduction to stove dynamics and chemistry-
Most stoves require vaporization of fuel for it to burn. Propane and butane fuel should already be pressurized (depending on the contester's temperature), but most multifuel stoves require priming and vaporization through either a generator tube or heat sink. Pressurized fuel is generally shot through a jet and through a tube where it mixes with oxygen before it hits a burner or diffusion plate. At 800-900°C larger hydrocarbons are depolymerised as C-C-bonds are cleaved by the heat (pyrolysis) to form carbon radicals (C-C-C-C → C-C• + •C-C). At 1150°C ethylene (C2H4) is striped of two of its hydrogen atoms (dehydrogenation), forming acetylene (H2C-CH2 → HC≡CH + H2), which is further decomposed into carbon and hydrogen (HC≡CH → 2C + H2). Hydrogen molecules are broken down into hydrogen atoms by thermal dissociation (H2 → H• + •H), react with O2 to form water (4H• + O2 → 2H2O + energy), which in turn releases energy that heats up surrounding carbon atoms. Heated carbon reacts with O2 to form CO. With sufficient levels of heat (1000°C) and O2, CO will combine with oxygen to form CO2 and release a great deal of the fuel's heat potential. If there is insufficient heat, O2, and/or disruption in the flame, you will get incomplete combustion with incandescence of the carbon (yellow flame) and release of CO with/without soot. Note: fuels with unsaturated hydrocarbons, branched species and aromatic compounds may need higher temperatures to fully pyrolyse.
For more information on stove chemistry and flame theory see How Backpacking Stoves Work.
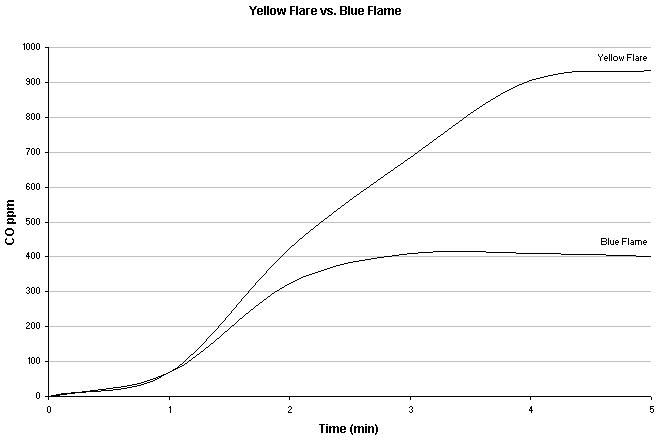
Notice dangerous levels of CO with yellow flare from a Coleman Peak stove with Coleman fuel. Source - Leigh-Smith S, Watt I, McFadyen A, et al. Comparison of carbon monoxide levels during heating of ice and water to boiling point with a camping stove. Wilderness Environ Med. 2004;15:164-170.
Yellow flames suggest incomplete combustions and is generally associated with production of large amounts of CO. If your stove gets yellow flames, you should consider shutting off your stove, possibly cleaning it (special attention given to the jet), replacing the fuel, and/or making sure it is sufficiently pressurized instead of subjecting yourself to what can be deadly levels of CO. Setting a stove at the highest output level that still produces blue flames produces the lowest levels of CO. Counter intuitively, setting a stove for low output, such as simmering, causes larger amounts of CO production than any other setting.
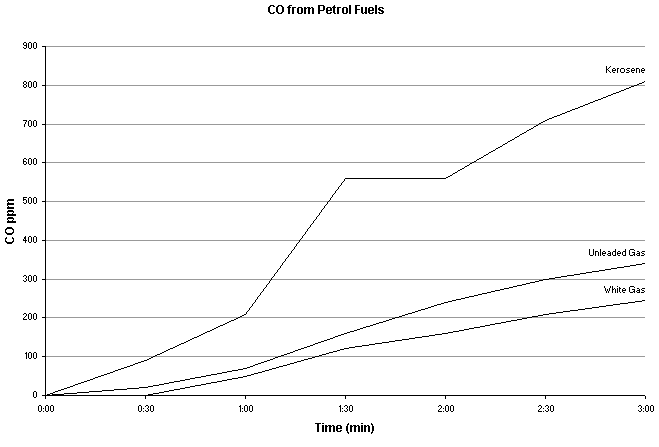
Schwartz RB, Ledrick DJ, Lindman AL. A comparison of carbon monoxide levels during the use of a multi-fuel camp stove. Wilderness Environ Med. 2001;12:236-238.
Stoves should be well maintained, clean, well pressurized (if applicable), and fed high quality fuel. One study of stove fuels (Schwartz RB, Ledrick DJ, Lindman AL. A comparison of carbon monoxide levels during the use of a multi-fuel camp stove. Wilderness Environ Med. 2001;12:236-238.) reported kerosene produced on average more than twice the level of CO than white or unleaded gas when used in a Sigg Fire Jet for 2 hours. Since kerosene is a "dirty" fuel with a higher content of unsaturated hydrocarbon chains and aromatic compounds than "cleaner" fuels, many of these compounds may not be fully pyrolysed leading to incomplete combustion and soot production.
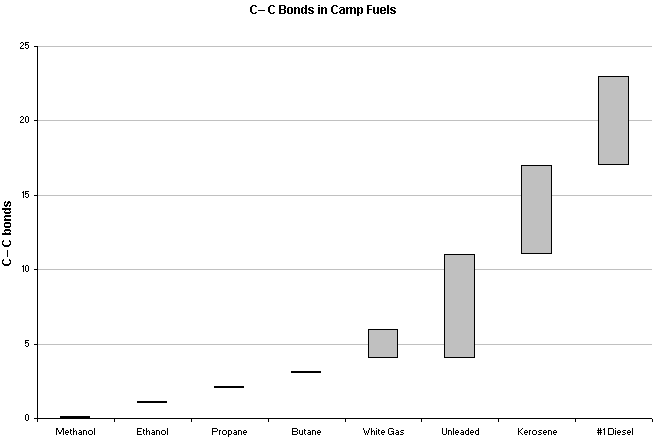
On average, kerosene has much larger molecules than white gas or unleaded gas, which may lead to incomplete breakdown of its hydrocarbons and lead to incomplete combustion. If the molecular size of fuel particles has an impact on CO production, then fuels such as alcohols and liquefied petroleum gasses should in theory produce less CO than even white gas. It is also possible that that size of the jet (which is paramount in fuel efficiency and limiting CO production) used in the test mentioned above may have been more suitable for white gas use than for kerosene, rendering the results and conclusions less useful but still very concerning. If you have a multifuel stove with interchangeable jets, you should ensure that you are using the proper jet for your fuel to maximize efficiency and decrease CO production. And on the subject of jets, a stove may not perform optimally at higher evaluations with a jet designed for sea level use due to decreased atmospheric pressures and available oxygen.
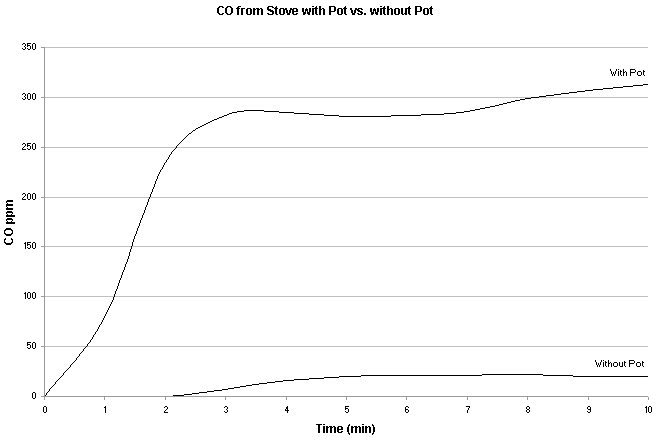
Notice dangerous levels of CO with yellow flare from a Coleman Peak stove with Coleman fuel. Source - Leigh-Smith S, Watt I, McFadyen A, et al. Comparison of carbon monoxide levels during heating of ice and water to boiling point with a camping stove. Wilderness Environ Med. 2004;15:164-170.
The more a flame is disrupted, the more CO is produced. Placing a pot, or anything else for that matter, on a stove drastically increased CO production. So relatively speaking to the effects of CO production, using a stove to heat a tent is far less dangerous than using it to melt snow or cook with.
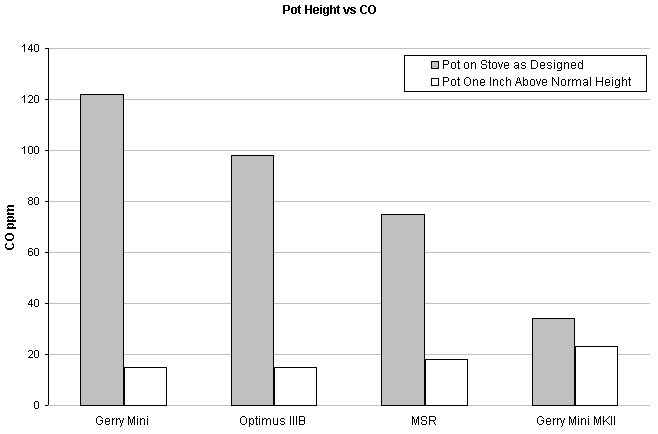
New Research Proves Backpacking Stoves More Deadly Than Suspected. Article summarized from "Backpacking Equipment Buyer's Guide", 1978 by William Kemsley & the editors of Backpacker Magazine
According to an article in the 1978 Backpacking Equipment Buyer's Guide, pots positioned above the flame will create less CO than pots placed in the flame (as most stoves are designed). The downside to designing a pot stand to elevate your pot higher above your stove is the possibility of decreased stability and fuel efficiency for cooking.
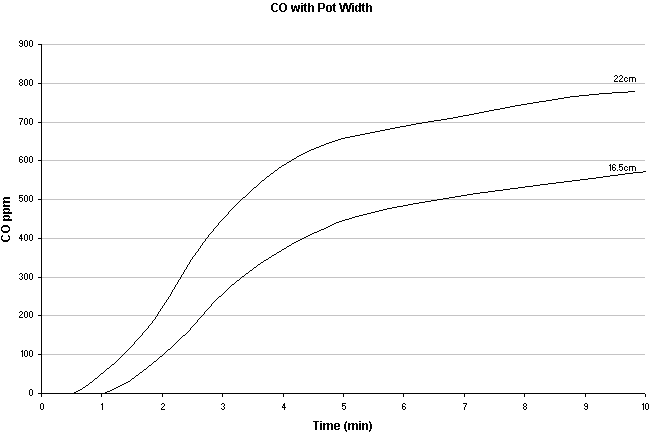
CO production differences with two different pot sizes with a Coleman Dual Fuel 533 and Coleman fuel. Leigh-Smith S, Stevenson R, Watt M, et al. Comparison of carbon monoxide levels during heating of water to boiling point with a camping stove using different diameter pans. Wilderness Environ Med. 2004;15:164-170.
Likewise, smaller diameter pots will create less CO than larger diameter pots.
| CO PPM | |||
| Simmer | Medium | High | |
| Micron Ti | 40 | 88 | 90 |
| Colman F1 UL | 75 | 154 | |
| Pocket rocket | 240 | 220 | 140 |
| Optimus Crux | 300 | 260 | |
| Snow Peak GS100 | 5 | 21 | |
| Vargo Jet Ti | 30 | 12 | 22 |
| Jetboil GCS | 5 | 6 | 90 |
tgomagazine.co.uk subject-primus-micron-ti-25
backpackinglight.com M Stoves, Tents and Carbon Monoxide - Deadly or Not?
Your stove also maters. Some stoves just produce more carbon monoxide than others. This can be due to poor burner design, improperly sized jets, pot height setting and overall lack of concern by the engineers and marketers producing a stove. As it is easier to sell a hot burning stove with a giant carbon monoxide stick on it than a not so hot burning stove with less odorless gas, it is up to the consumers to perform their own research on stove safety and to change the market priorities.
In summary-
Those wanting to maximize the dangers or CO poisoning should use kerosene in a dirty and inadequately pressurized multifuel stove set on simmer with a very wide pot set directly on top of their stove while in a snow cave with minimal ventilation at a very high altitude.
Those who wish to decrease the dangers of CO poisoning should avoid stove/lantern use in confined areas all together or at the very least observe the following:
|
Risk factors for CO poisoning and recommendations for avoidance
|
|
|
Risk factor for CO poisoning |
Recommendation for avoidance |
|
Cooking |
Avoid prolonged simmering Keep stove highly pressurized Use a maximum blue flame and avoid low flames Use small-diameter pans Keep pot out of flame Use white pure fuels |
|
Yellow flame |
Turn stove off, repressurize, relight Maximum tent ventilation for few min |
|
Inadequate ventilation causing:
|
Ventilation area at least 50 cm2 Ventilation CO egress port as high as possible Ventilation O2 ingress port sited low Avoid minimal ventilation which paradoxically elevates CO concentration Note higher CO risk in tents in zero-wind conditions |
|
Insidious onset if sedentary |
Beware headache and tachycardia Regular trips outside to unmask symptoms |
|
Duration of CO exposure |
|
|
Stale air in tents (low O2) |
Ventilate tent at regular intervals Ventilation does not have to be continuous |
|
Dehydration |
Good hydration |
|
Snow holes are worse than tents |
Attention to above recommendations |
|
Altitude |
|
|
Hyperventilation |
|
|
Tent icing and snow cover |
Attempt to keep tent fabric porous by regular clearing |
|
Modified version of table in Leigh-Smith S. Carbon monoxide poisoning in tents--a review. Wilderness and Environmental Medicine. 2004 Fall;15(3):157-63. |
|
A lot of effort has gone into determining the level of risk with exposure to CO produced by stoves, but it still isn't clear how much exposure is actually safe. Fist off, most research is limited to CO levels in the air and its effect on COHb levels in the blood. Although CO bound to hemoglobin is the main cause of asphyxiation, most research fails to consider the other effects of CO, such as its direct neurotoxicity and affects of CO bound to non-hemoglobin proteins. Since cumulative exposure to CO can cause very serious neurological, psychiatric, cardiac and other problems without noticeable impact on COHb levels, this is a considerable oversight in evaluating CO dangers.
Good information on the affects of CO at altitude is also important, but difficult and dangerous to determine. It is quite conceivable that CO exposure at high altitudes is much more dangerous than speculated.
Pertinent to stove use in tents and shelters, it is important to note that little effort has gone into comparing various stove designs, configurations and fuels. The significant variation in stove and fuel performance is well know to stovers, but good CO information pertaining to each particular setup is truly lacking. Most of the research has been limited to kerosene and white gas stoves without consideration to brand/design differences, performance at altitude, or other fuels (butane, propane, methanol, ethanol, etc.).
Notable Stove Research
|
Research |
Year |
Stoves and/or Fuel Used |
|
Irving L, Scholander P, Edwards G. |
1942 |
Primus |
|
Pugh L. |
1959 |
Primus |
|
Turner WA, Cohen MA, Moore S, et al.. |
1988 |
Optimus 111B, Optimus 8R, MSR Firefly |
|
Harrigan M. |
1992 |
Coleman Peak stove |
|
Keyes LE, Hamilton RS, Rose JS. |
2001 |
Stove type decided by subjects. |
|
Schwartz RB, Ledrick DJ, Lindman AL. |
2001 |
Sigg Fire Jet - white gas, unleaded and kerosene |
|
Source - Leigh-Smith S, Watt I, McFadyen A, et al. |
2004 |
Coleman Peak stove with Coleman fuel. |
|
Leigh-Smith S, Stevenson R, Watt M, et al. |
2004 |
Coleman Dual Fuel 533 and Coleman fuel. |
|
Thomassen O, Brattebo G, Rostrup M. |
2004 |
Optimus 111 |
Further backpacking/climbing CO research should include comparisons of different fuel types, stoves, jet sizes and other configurations (pot height and windscreen height/diameter/ventilation) as well as more comprehensive information on affects of altitude and long term effects of low level CO exposure generally encountered by trekkers.
Note: Since publication of this page, Roger Caffin has released the Stoves, Tents and Carbon Monoxide - Deadly or Not? Series which discusses carbon monoxide concerns for many stoves used by backpackers and climbers.
The EPA has also published:
Smith KR, Uma R, Kishore VVN, Lata K, Joshi V, Zhang J, Rasmussen RA, Khalil MAK (2000) Greenhouse Gases from Small-scale Combustion Devices in Developing Countries, Phase IIa: Household Stoves in India. EPA-600/R-00-052, U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC. Link.
This article compares various real world stoves used in developing countries.
Leigh-Smith S. Carbon monoxide poisoning in tents--a review. Wilderness and Environmental Medicine. 2004 Fall;15(3):157-63.
This review is a must read and is the paramount source of most of the following references and much of the information on this page-
1. Cohen MA. Air pollution exposures to campers inside of tents: A study of the use of camping stoves and lanterns. Proceedings International Specialty Conference Indoor Air Quality Cold Climates. Ottawa, Ontario, Canada; May 1985.
2. Foutch RG, Henrichs W. Carbon monoxide poisoning at high altitudes. Am J Emerg Med. 1988;6:596-598.
3. Byrd R. Alone. New York, NY: Adventure Library; 1938.
4. Stefansson W. Unsolved Mysteries of the Arctic. New York, NY: Macmillan; 1939.
5. Irving L, Scholander P, Edwards G. Experiments on carbon monoxide poisoning in tents and snow houses. J Ind Hyg Toxicol. 1942;24:213.
6. Pugh L. Carbon monoxide hazard in Antarctica. BMJ. 1959;34(5116):192-196.
7. Girman JR, Chang YL, Hayward SB, et al. Causes of unintentional deaths from carbon monoxide poisonings in California. West J Med 1998;168:158-165.
8. Rupp W,Nadjem H, Thoma KH. Alcohol stove as a source of CO poisoning in a camper [in German]. Arch Kriminol. 2000;206:8-13.
9. Centers for Disease Control and Prevention. Carbon monoxide poisoning deaths associated with camping-Georgia, March 1999. JAMA. 1999;282:1326.
10. Schwartz RB, Ledrick DJ, Lindman AL. A comparison of carbon monoxide levels during the use of a multi-fuel camp stove. Wilderness Environ Med. 2001;12:236-238.
11. Seibert R. Climbs and expeditions-Alaska. Am Alpine J. 1986;28:139-142.
12. Krakauer J. Into Thin Air-A Personal Account of the Everest Disaster. Chatham, UK: Pan Books; 1997.
13. Coburn RF, Blakemore WS, Forster RE. Endogenous carbon monoxide production in man. J Clin Invest. 1963;42: 1172-1178.
14. Stewart RD. The effect of carbon monoxide on humans. Ann Rev Pharm. 1975;15:409-423.
15. Dubois L, Zdrojewski A, Monkman JL. The analysis of carbon monoxide in urban air at the ppm level, and the normal carbon monoxide value. J Air Pollut Control Assoc. 1966;16:135-139.
16. Heath and Safety Executive, UK Government. Carbon monoxide: health hazards and precautionary measures. Guidance Note. 2nd ed. Sudbury, Ontario, Canada: HSE Books; 1998.
17. Stewart RD, Peterson IE, Baretta ED, et al. Experimental human exposure to carbon monoxide. Arch Environ Health. 1970;21:154-164.
18. Astrup P. Intraerythrocytic 2,3-diphosphoglycerate and carbon monoxide exposure. Ann N Y Acad Sci. 1970;174: 252-254.
19. Thomas MF, Penney DG. Hematologic responses to carbon monoxide and altitude: a comparative study. J Appl Physiol. 1977;43:365-369.
20. Coburn RF, Forster RE, Kane PE. Considerations of the physiological variables that determine the blood carboxyhaemoglobin concentration in man. J Clin Invest. 1965; 44:1899-1910.
21. Rylander R, Vesterlund J. Carbon monoxide criteria. With reference to effects on the heart, central nervous system and fetus. Scand J Work Environ Health. 1981;7(suppll): 1-39.
22. Henderson Y, Turner J. Carbon monoxide as a hazard of polar exploration. Nature. 1940;145:92-95.
23. Leigh-Smith S, Watt I, McFadyen A, et al. Comparison of carbon monoxide levels during heating of ice and water to boiling point with a camping stove. Wilderness Environ Med. 2004;15:164-170.
24. Turner WA, Cohen MA, Moore S, et al. Carbon monoxide exposure in mountaineers on Denali. Alaska Med. 1988; 30:85-90.
25. Harrigan M. A Study of Carbon Monoxide Exposure Amongst Troops During Arctic Training [Master's thesis], 1992.
26. Keyes LE, Hamilton RS, Rose JS. Carbon monoxide exposure from cooking in snow caves at high altitude. Wilderness Environ Med.2001;12:208-212.
27. Westerlung K, von Ubisch H. Carbon monoxide from small camping appliances and from stoves without chimney connection. Nordisk Hygienisk Tidskrift. 1972;53:26- 33.
28. Prescher KE. Occurrence of carbon monoxide, carbon dioxide and nitrogen oxides during the use of gas stoves [in German]. Schriftenr Ver Wasser Boden Lufthyg. 1982;53: 191-198.
29. Leigh-Smith S, Stevenson R, Watt M, et al. Comparison of carbon monoxide levels during heating of water to boiling point with a camping stove using different diameter pans. Wilderness Environ Med. 2004;15:164-170.
30. Sokal JA, Kralkowska E. The relationship between exposure duration, carboxyhemoglobin, blood glucose, pyruvate and lactate and the severity of intoxication in 39 cases of acute carbon monoxide poisoning in man. Arch Toxicol. 1985;57:196-199.
31. Vollmer E, King G, Birren I. The effects of carbon monoxide on three types of performance at simulated altitudes of 10000 and 15000 feet. J Exp Psychol.1946;36:244- 251.
32. Altitude as a factor in air pollution. Research Triangle Park, NC: Environmental Protection Agency; 1978. U.S. EPA No. 600/9-78-015. Monograph.
33. Forbes WH, Sargent F, Houghton FJW. The rate of carbon monoxide uptake by normal men. Am J Physiol. 1945;143: 594-608.
34. McGrath JI. Effects of altitude on endogenous carboxyhemoglobin levels. J Toxicol Environ Health. 1992;35: 127-133.
35. Ellenhorn M. Diagnosis and treatment of human poisoning. In: Ellenhorn MJ, Schonwald S, Ordog G, Wasserperger J, eds. Ellenhorn's Medical Toxicology. Baltimore, MD: Williams and Wilkins; 1997.
36. Leaf DA, Kleinman MT. Urban ectopy in the mountains: carbon monoxide exposure at high altitude. Arch Environ Health. 1996;51:283-290.
37. Collier C, Goldsmith J. Interactions of carbon monoxide at altitude. Atmos Environ. 1983;17:723-728.
38. Carbon Monoxide - Myths and Facts - Stove FAQ web page set up by Roger Caffin
Information based real world experiences in addition to flame chemistry and common sense.
39. New Research Proves Backpacking Stoves More Deadly Than Suspected. Article summarized from "Backpacking Equipment Buyer's Guide", 1978 by William Kemsley & the editors of Backpacker Magazine
Raising the pans on a stove decreases the amount of CO produced. At altitudes above 10,000 feet carbon monoxide poses a potentially dangerous hazard by reducing the already low oxygen saturation of the blood. The effects would depend also on the performance of the particular stove, the ventilation of the tent and the duration of cooking time.
40. Thomassen O, Brattebo G, Rostrup M.
Carbon monoxide poisoning while using a small cooking stove in a tent.
Am J Emerg Med. 2004 May;22(3):204-6.
Kerosene camping stoves produce CO concentrations high enough to cause
significant COHb levels in venous blood after 120 minutes' stay in the
tent.
41. Min SK.
A brain syndrome associated with delayed neuropsychiatric sequelae
following acute carbon monoxide intoxication. Acta Psychiatr Scand.
1986 Jan;73(1):80-6.
42. Peterson JE, Stewart RD: Predicted the carboxy-hemoglobin levels
result from carbon monoxide exposure. J Appl Physiol 1975;39:633-638.
43. Tannheimer M, Thomas A, Gerngross H. Oxygen saturation course and altitude symptomatology during an expedition to broad peak (8047 m). Int J Sports Med. 2002 Jul;23(5):329-35.
44. Hampson NB, Mathieu D, Piantadosi CA, Thom SR, Weaver LK. Carbon monoxide poisoning: interpretation of randomized clinical trials and unresolved treatment issues. Undersea Hyperb Med. 2001 Fall;28(3):157-64.
45. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998 Nov 26;339(22):1603-8.